Subtitle
FLASH radiotherapy for the treatment of symptomatic bone metastases in the thorax (FAST-02): protocol for a prospective study of a novel radiotherapy approach.
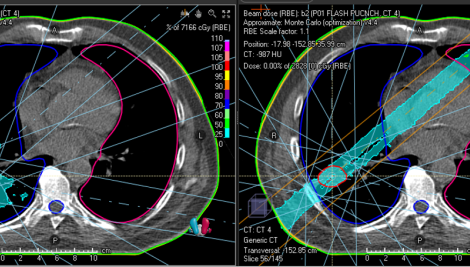
This is a trial protocol of FAST-02. This trial includes 10 patients with 1-3 painful bone metastases in the thorax, excluding bone metastases in the spine. Treatment will be 8 Gy in a single fraction administered at ≥ 40 Gy/s on a FLASH-enabled proton therapy system delivering a single transmission proton beam. Primary study endpoints are efficacy (pain relief) and safety. Registration: ClinicalTrials.gov NCT05524064.